For an updated version of these pages, click here


Substitution preferences:
All protein types:
| Favoured | Ala ( 1) | Asn ( 1) | Thr ( 1) | |||||
| Neutral | Glu ( 0) | Lys ( 0) | Gly ( 0) | Gln ( 0) | Asp ( 0) | |||
| Disfavoured | Arg (-1) | Met (-1) | His (-1) | Pro (-1) | Cys (-1) | Leu (-2) | Val (-2) | Phe (-2) |
| Tyr (-2) | Ile (-2) | Trp (-3) |
| Favoured | ||||||||
| Neutral | Cys ( 0) | Asp ( 0) | Glu ( 0) | Lys ( 0) | Gly ( 0) | His ( 0) | Asn ( 0) | Pro ( 0) |
| Gln ( 0) | Arg ( 0) | Ala ( 0) | Thr ( 0) | |||||
| Disfavoured | Val (-1) | Tyr (-1) | Met (-1) | Phe (-2) | Trp (-2) | Ile (-2) | Leu (-2) |
| Favoured | Thr ( 1) | |||||||
| Neutral | Pro ( 0) | Asp ( 0) | Glu ( 0) | Asn ( 0) | Gly ( 0) | His ( 0) | Lys ( 0) | Arg ( 0) |
| Ala ( 0) | Gln ( 0) | |||||||
| Disfavoured | Ile (-1) | Met (-1) | Leu (-1) | Val (-1) | Trp (-1) | Tyr (-1) | Phe (-2) | Cys (-5) |
| Favoured | Asn ( 2) | Thr ( 2) | Ala ( 2) | Cys ( 1) | Gly ( 1) | |||
| Neutral | Glu ( 0) | Tyr ( 0) | Asp ( 0) | |||||
| Disfavoured | Pro (-1) | Gln (-1) | Phe (-1) | Lys (-1) | Arg (-1) | Val (-1) | Ile (-1) | His (-2) |
| Leu (-2) | Met (-2) | Trp (-3) |
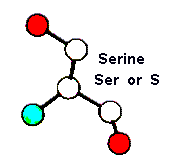
Role in structure: Being a fairly indifferent amino acid, Serine can reside both within the interior of a protein, or on the protein surface. Its small size means that it is relatively common within tight turns on the protein surface, where it is possible for the Serine side-chain hydroxyl oxygen to form a hydrogen bond with the protein backbone, effectively mimicking Proline.
Role in function: Serines are quite common in protein functional centres. The hydroxyl group is fairly reactive, being able to form hydrogen bonds with a variety of polar substrates.
Perhaps the best known role for Serine in protein active sites is found in the classical Asp-His-Ser catalytic triad found in many hydrolases (e.g. proteases, lipases, etc.). Here, a Serine, aided by a Histidine and an Aspartate acts as a nucleophile to hydrolyse (effectively cut) other molecules.
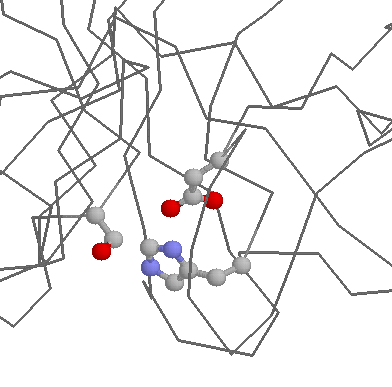
A common role for Serines (and Threonines and Tyrosines) within intracellular proteins is phosphorylation. Protein kinases frequently attach phosphates to Serines in order to fascilitate the signal transduction process. Note that in this context, Serine can often be replaced by Threonine, but is unlikely to be replaced by Tyrosine, as the enzymes that catalyse the reactions (i.e. the protein kinases) are highly specific (i.e. Tyrosine kinases generally do not work on Serines/Threonines and vice versa).