For an updated version of these pages, click here

Substitution preferences:
All protein types:
| Favoured | ||||||||
| Neutral | Ala ( 0) | |||||||
| Disfavoured | Ile (-1) | Val (-1) | Met (-1) | Leu (-1) | Ser (-1) | Thr (-1) | Trp (-2) | Phe (-2) |
| Tyr (-2) | Gly (-3) | His (-3) | Lys (-3) | Gln (-3) | Arg (-3) | Asn (-3) | Pro (-3) | |
| Asp (-3) | Glu (-4) |
| Favoured | ||||||||
| Neutral | Ala ( 0) | Thr ( 0) | Val ( 0) | Phe ( 0) | Tyr ( 0) | His ( 0) | Ile ( 0) | Met ( 0) |
| Leu ( 0) | Ser ( 0) | |||||||
| Disfavoured | Lys (-1) | Asn (-1) | Gly (-1) | Trp (-1) | Arg (-1) | Pro (-2) | Asp (-2) | Gln (-2) |
| Glu (-2) |
| Favoured | ||||||||
| Disfavoured | Ala (-4) | Tyr (-4) | Val (-4) | Thr (-5) | Trp (-5) | Phe (-5) | His (-5) | Ile (-5) |
| Leu (-5) | Met (-5) | Gln (-5) | Arg (-5) | Ser (-5) | Gly (-6) | Glu (-6) | Pro (-6) | |
| Lys (-6) | Asn (-6) | Asp (-7) |
| Favoured | Tyr ( 3) | Ser ( 2) | Phe ( 1) | Trp ( 1) | ||||
| Neutral | Ala ( 0) | Thr ( 0) | Val ( 0) | |||||
| Disfavoured | Asn (-1) | Gly (-1) | His (-1) | Arg (-1) | Ile (-1) | Leu (-1) | Met (-1) | Pro (-3) |
| Gln (-3) | Lys (-3) | Glu (-3) | Asp (-3) |
Role in structure: The role of Cysteines in structure is very dependent on the cellular location of the protein in which they are contained. Within extracellular proteins, cysteines are frequently involved in disulphide bonds, where pairs of cysteines are oxidised to form a covalent bond. These bonds serve mostly to stabilise the protein structure, and the structure of many extracellular proteins is almost entirely determined by the topology of multiple disulphide bonds (these are generally classified as small disulphide-rich proteins; and example is shown below).
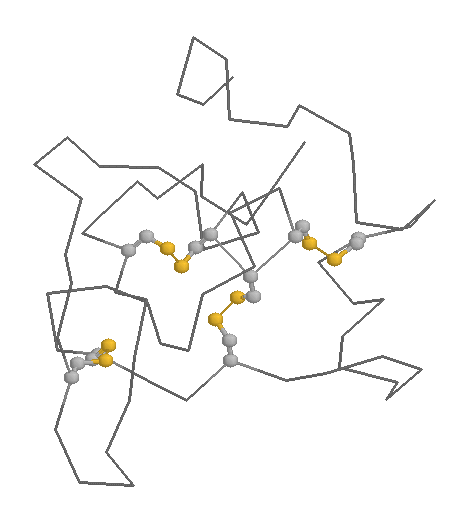
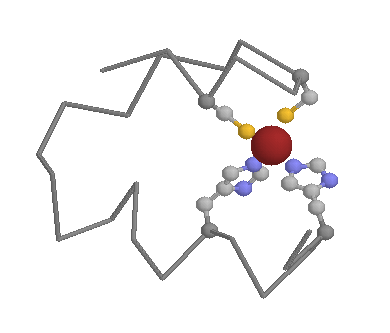
In the intracellular environment Cysteines can still play a key structural role. Their sulfydryl side-chain is excellent for binding to metals, such as zinc, meaning the Cysteines (and other amino acids such as Histidines) are very common in metal binding motifs such as zinc fingers (below).
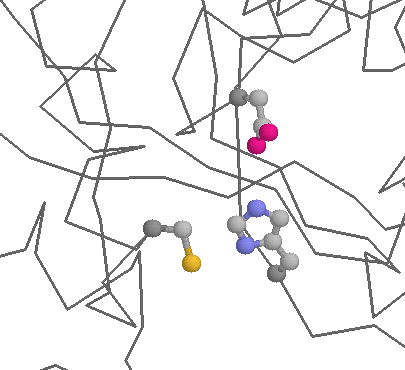
Role in function: Cysteines are also very common in protein active and binding sites. Binding to metals (see above) can also be important in enzymatic functions (e.g. metal proteases). Cysteine can also function as a nucleophile (i.e. the reactive centre of an enzyme). Probably the best known example of this occurs within the Cysteine proteases, such as caspases, or papains (below), where Cysteine is the key catalytic residue, being helped by a Histidine and an Asparagine.