For an updated version of these pages, click here


Substitution preferences:
All protein types:
| Favoured | Glu ( 2) | Asn ( 1) | ||||||
| Neutral | Ser ( 0) | Gln ( 0) | ||||||
| Disfavoured | Gly (-1) | His (-1) | Lys (-1) | Pro (-1) | Thr (-1) | Ala (-2) | Arg (-2) | Phe (-3) |
| Met (-3) | Val (-3) | Ile (-3) | Tyr (-3) | Cys (-3) | Leu (-4) | Trp (-4) |
| Favoured | Glu ( 1) | Asn ( 1) | ||||||
| Neutral | Thr ( 0) | Lys ( 0) | Gly ( 0) | His ( 0) | Pro ( 0) | Gln ( 0) | Arg ( 0) | Ser ( 0) |
| Disfavoured | Ala (-1) | Cys (-2) | Val (-2) | Trp (-2) | Tyr (-2) | Met (-2) | Ile (-3) | Leu (-3) |
| Phe (-3) |
| Favoured | Asn ( 1) | |||||||
| Neutral | Thr ( 0) | Glu ( 0) | Lys ( 0) | Gly ( 0) | His ( 0) | Pro ( 0) | Gln ( 0) | Arg ( 0) |
| Ser ( 0) | ||||||||
| Disfavoured | Ala (-1) | Val (-1) | Met (-2) | Leu (-2) | Phe (-2) | Ile (-2) | Tyr (-2) | Trp (-3) |
| Cys (-7) |
| Favoured | Glu ( 8) | Asn ( 6) | His ( 3) | Lys ( 3) | Gly ( 2) | Gln ( 2) | Arg ( 1) | |
| Neutral | Thr ( 0) | Ala ( 0) | Ser ( 0) | |||||
| Disfavoured | Tyr (-2) | Pro (-2) | Cys (-3) | Ile (-3) | Val (-3) | Met (-3) | Trp (-4) | Leu (-5) |
| Phe (-6) |
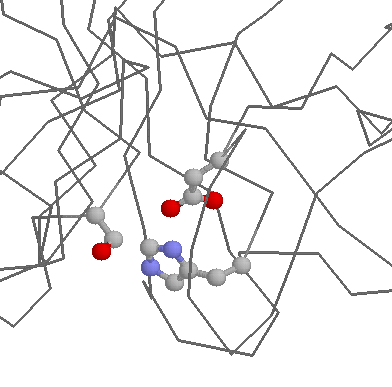
Role in structure: Being charged and polar, Asparates prefer generally to be on the surface of proteins, exposed to an aqueous environment. When buried within the protein, Aspartates (and Glutamtes) are frequently involved in salt-bridges, where they pair with a positively charged amino acid (such as Arginine shown below) to create stabilising hydrogen bonds, that can be important for protein stability.
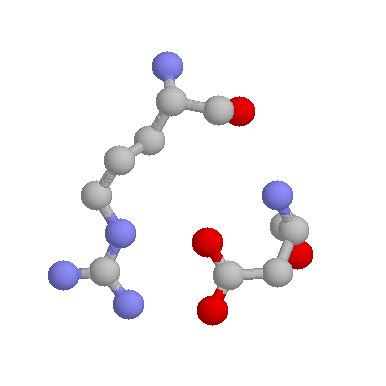
Aspartate has a shorter side-chain than the very similar Glutamate meaning that is slightly more rigid within protein structures. This gives it a slightly stronger preference to be involved in protein active sites. Probably the most famous example of Asparate being involved in an active site is found within Serine proteases such as Trypsin, where it functions in the classical Asp-His-Ser catalytic triad: