For an updated version of these pages, click here


Substitution preferences:
All protein types:
| Favoured | Tyr ( 2) | Asn ( 1) | ||||||
| Neutral | Glu ( 0) | Gln ( 0) | Arg ( 0) | |||||
| Disfavoured | Lys (-1) | Ser (-1) | Asp (-1) | Phe (-1) | Met (-2) | Thr (-2) | Trp (-2) | Pro (-2) |
| Ala (-2) | Gly (-2) | Cys (-3) | Ile (-3) | Leu (-3) | Val (-3) |
| Favoured | Tyr ( 1) | |||||||
| Neutral | Cys ( 0) | Asp ( 0) | Glu ( 0) | Asn ( 0) | Lys ( 0) | Gln ( 0) | Arg ( 0) | Ser ( 0) |
| Thr ( 0) | Trp ( 0) | |||||||
| Disfavoured | Phe (-1) | Gly (-1) | Met (-1) | Val (-1) | Leu (-1) | Pro (-1) | Ala (-1) | Ile (-2) |
| Favoured | ||||||||
| Neutral | Ala ( 0) | Pro ( 0) | Asn ( 0) | Asp ( 0) | Glu ( 0) | Gly ( 0) | Lys ( 0) | Gln ( 0) |
| Arg ( 0) | Ser ( 0) | Thr ( 0) | Tyr ( 0) | |||||
| Disfavoured | Ile (-1) | Met (-1) | Leu (-1) | Val (-1) | Trp (-1) | Phe (-1) | Cys (-5) |
| Favoured | Gln ( 7) | Tyr ( 6) | Arg ( 5) | Lys ( 4) | Asp ( 3) | Asn ( 3) | Glu ( 2) | |
| Disfavoured | Trp (-1) | Cys (-1) | Ser (-2) | Thr (-2) | Phe (-3) | Ala (-3) | Gly (-3) | Met (-3) |
| Ile (-4) | Pro (-4) | Leu (-4) | Val (-4) |
Role in structure: Histidine has a pKa near to that of physiological pH, meaning that it is relatively easy to move protons on and off of the side chain (i.e. changine the side chain from neutral to postive charge). This flexibility has two effects. The first is that it means Histidines is rather ambiguous about whether it prefers to be buried in the protein core, or exposed to solvent. The second is that it is an ideal residue for protein functional centres (discussed below).
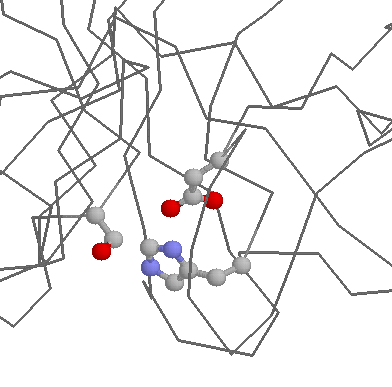
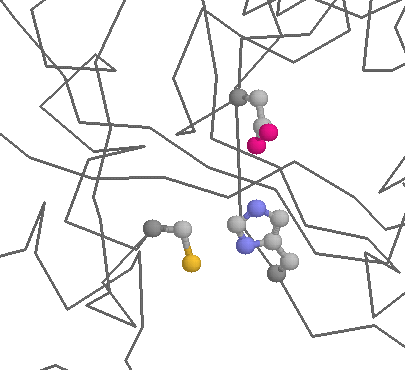
Role in function: Histidines are the most common amino acids in protein active or binding sites. They are very common in metal binding sites (e.g. zinc), often acting together with Cysteines or other amino acids:
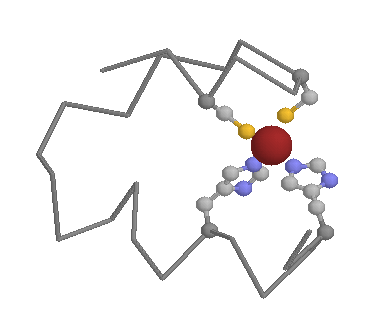
The ease with which protons can be transfered on and off of Histidines makes them ideal for charge relay systems such as those found within catalytic triads, found in many Cysteine and Serine proteases: