For an updated version of these pages, click here


Substitution preferences:
All protein types:
| Favoured | Lys ( 2) | Gln ( 1) | ||||||
| Neutral | Asn ( 0) | Glu ( 0) | His ( 0) | |||||
| Disfavoured | Ala (-1) | Ser (-1) | Thr (-1) | Met (-1) | Asp (-2) | Gly (-2) | Leu (-2) | Tyr (-2) |
| Pro (-2) | Val (-3) | Trp (-3) | Phe (-3) | Cys (-3) | Ile (-3) |
| Favoured | Lys ( 1) | |||||||
| Neutral | Asp ( 0) | Glu ( 0) | Asn ( 0) | Gly ( 0) | His ( 0) | Met ( 0) | Pro ( 0) | Gln ( 0) |
| Ala ( 0) | Ser ( 0) | Thr ( 0) | ||||||
| Disfavoured | Cys (-1) | Ile (-1) | Val (-1) | Trp (-1) | Tyr (-1) | Leu (-1) | Phe (-2) |
| Favoured | Lys ( 1) | |||||||
| Neutral | Asn ( 0) | Asp ( 0) | Glu ( 0) | Met ( 0) | Gly ( 0) | His ( 0) | Ile ( 0) | Ala ( 0) |
| Gln ( 0) | Pro ( 0) | Ser ( 0) | Thr ( 0) | Val ( 0) | Tyr ( 0) | |||
| Disfavoured | Phe (-1) | Trp (-1) | Leu (-1) | Cys (-5) |
| Favoured | Lys ( 9) | Gln ( 6) | Trp ( 5) | His ( 5) | Asn ( 2) | Glu ( 2) | Asp ( 1) | |
| Neutral | Gly ( 0) | Met ( 0) | ||||||
| Disfavoured | Tyr (-1) | Ala (-1) | Ser (-1) | Thr (-1) | Cys (-1) | Val (-2) | Pro (-3) | Ile (-3) |
| Leu (-3) | Phe (-4) |
Note that a change from Arginine to Lysine is not always neutral. In certain structural or functional contexts, such a mutation can be devestating to protein stability of function (see below).
Role in structure: Arginine frequently plays an important role in structure. First, it can be considered to be somewhat amphipathic as the part of the side chain nearest to the backbone is long, carbon containing and hydrophobic, whereas the end of the side chain is positively charged. For this reason, one can find Arginines where part of the side-chain is buried, and only the charged portion is on the outside of the protein. However, this is by no means always the case, and generally Arginines prefer to be on the outside of proteins.
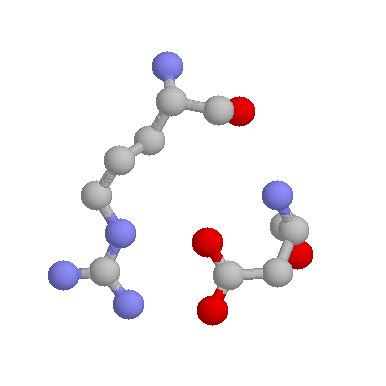
Arginines are also frequently involved in salt-bridges, where they pair with a negatively charged amino acid (such as Aspartate, shown below) to create stabilising hydrogen bonds, that can be important for protein stability.
It contains a complex guanidinium group on its side-chain that has a geometry and charge distribution that is ideal for binding negatively charged groups on phosphates (it is able to form multiple hydrogen bonds) A good example can be found in the src homology 2 (SH2) domains:
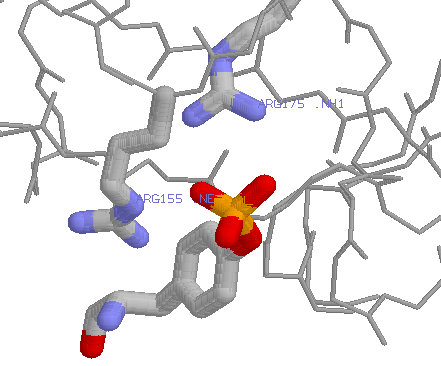
Note that in this context Arginine is not easily replaced by Lysine. Although Lysine can interact with phosphates, it contains only a single amino group, meaning it is more limited in the number of hydrogen bonds it can form. A change from Arginine to Lysine in some contexts can thus be disasterous.