For an updated version of these pages, click here


Substitution preferences:
All protein types:
| Favoured | Ser ( 1) | His ( 1) | Asp ( 1) | |||||
| Neutral | Thr ( 0) | Glu ( 0) | Lys ( 0) | Gly ( 0) | Gln ( 0) | Arg ( 0) | ||
| Disfavoured | Ala (-2) | Met (-2) | Tyr (-2) | Pro (-2) | Phe (-3) | Val (-3) | Ile (-3) | Leu (-3) |
| Cys (-3) | Trp (-4) |
| Favoured | Asp ( 1) | |||||||
| Neutral | Thr ( 0) | Glu ( 0) | Lys ( 0) | Gly ( 0) | His ( 0) | Gln ( 0) | Arg ( 0) | Ser ( 0) |
| Disfavoured | Ala (-1) | Cys (-1) | Pro (-1) | Tyr (-1) | Met (-1) | Leu (-2) | Val (-2) | Trp (-2) |
| Phe (-2) | Ile (-2) |
| Favoured | Asp ( 1) | |||||||
| Neutral | Ala ( 0) | Glu ( 0) | Thr ( 0) | Gly ( 0) | His ( 0) | Lys ( 0) | Pro ( 0) | Gln ( 0) |
| Arg ( 0) | Ser ( 0) | |||||||
| Disfavoured | Met (-1) | Ile (-1) | Val (-1) | Tyr (-1) | Phe (-2) | Leu (-2) | Trp (-3) | Cys (-6) |
| Favoured | Asp ( 6) | Lys ( 5) | Gln ( 3) | His ( 3) | Ser ( 2) | Arg ( 2) | Glu ( 1) | Thr ( 1) |
| Disfavoured | Ala (-1) | Tyr (-1) | Cys (-1) | Pro (-2) | Gly (-2) | Met (-2) | Trp (-3) | Val (-3) |
| Ile (-3) | Leu (-4) | Phe (-4) |
Role in structure: Being polar, Asparagine prefers generally to be on the surface of proteins, exposed to an aqueous environment.
Role in function: Asparagines are quite frequently involved in protein active or binding sites. The polar side-chain is good for interactions with other polar or charged atoms.
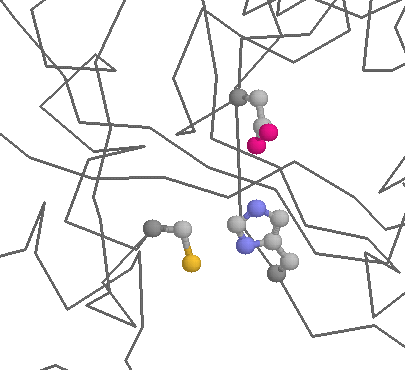
Asparagine can play a similar role to Aspartate in some proteins. Probably the best example is found in certain Cysteine proteases, where it forms part of the Asn-His-Cys catalytic triad: